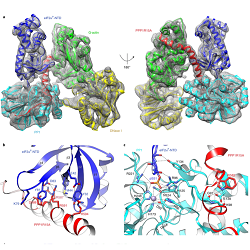
Like many key biological pathways, the Integrated Stress Response (ISR) is carefully regulated. A known molecular trigger for the ISR is the stress-induced phosphorylation of the α-subunit of translation initiation factor 2 (eIF2αP) at Ser-51. Dephosphorylation of this residue has a converse effect on the ISR, and is therefore equally important to regulation. However, this latter step is less well understood than the activating event – in large part because of the more complex nature of protein phosphatases, whereby substrate specificity is conferred to a catalytic core subunit by accessory proteins in a holoenzyme complex. Publishing in Nature Structural & Molecular Biology, CIMR’s Yahui Yan, Heather Harding and David Ron provide a structural basis of how the ISR is switched off through the dephosphorylation of phospho-eIF2αP by a PP1/PPP1R15A/G-actin trimeric holophosphatase. Beyond insights into the fundamentals of protein dephosphorylation, this new knowledge could also guide future efforts to target ISR regulation pharmacologically.

