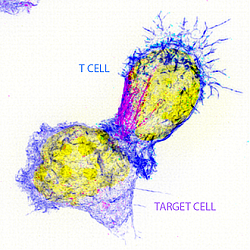
Barton PR, Davenport AJ, Hukelmann J, Cantrell DA, Stinchcombe JC and Griffiths GM. Super-killer CTLs are generated by single gene deletion of Bach2. European Journal of Immunology, Epub ahead of print. doi: 10.1002/eji.202249797 (2022).
Lisci M, Griffiths GM. Arming a killer: mitochondrial regulation of CD8+ T cell cytotoxicity. Trends in Cellular Biology, Epub ahead of print. doi: 10.1016/j.tcb.2022.05.007 (2022).
Richard AC, Frazer G, Ma CY, Griffiths GM. Staggered starts in the race to T cell activation. Trends in Immunology, 42(11):994-1008. doi: 10.1016/j.it.2021.09.004 (2021)
Frazer G, Gawden-Bone CM, Dieckmann NMG, Asano Y and Griffiths GM. Signal strength controls the rate of polarization within CTLs during killing. Journal of Cell Biology, 220 (10): e202104093 (2021).
Lisci M, Barton PR, Randzavola LR, Ma CY, Marchingo JM, Cantrell DA, Paupe V, Prudent J, Stinchcombe JC and Griffiths GM. Mitochondrial translation is required for sustained killing by cytotoxic T cells. Science, 15;374(6565):eabe9977. doi: 10.1126/science.abe9977 (2021).
Ma, C, Marioni, J, Griffiths, GM and Richard, A. Stimulation strength controls the rate of initiation but not the molecular organization of TCR-induced signalling. eLife, 9:e53948. doi: 10.7554/eLife.53948 (2020).
Randzavola LO, Strege K, Juzans M, Asano Y, Stinchcombe JC, Gawden-Bone CM, Seaman MNJ, Kuijpers T. and Griffiths GM. Loss of ARPC1B impairs cytotoxic T lymphocyte maintenance and cytolytic activity. Journal of Clinical Investigation, 129(12), pp.5600-5614 (2019).
Ritter AT, Kapnick SM, Murugesan S, Schwartzberg PL, Griffiths GM, Lippincott-Schwartz J. Cortical actin recovery at the immunological synapse leads to termination of lytic granule secretion in cytotoxic T lymphocytes. Proc. Natl Acad. Sci. USA 114(32):E6585-E6594. doi: 10.1073/pnas.1710751114 (2017).
Stinchcombe JC, Randzavola L, Angus KL, Mantell JM, Verkade P & Griffiths GM. Mother Centriole Distal Appendages Mediate Centrosome Docking at the Immunological Synapse and Reveal Mechanistic Parallels with Ciliogenesis. Current Biol. 25, 3239–3244 (2015).
Ritter AT, Asano Y, Stinchcombe JC, Dieckmann NM, Chen BC, Gawden-Bone C, van Engelenburg S, Legant W, Gao L, Davidson MW, Betzig E, Lippincott-Schwartz J, Griffiths GM. Actin depletion initiates events leading to granule secretion at the immunological synapse. Immunity 42, 864-876. doi: 10.1016/j.immuni.2015.04.013 (2015).
Jenkins, M. R. et al. Distinct structural and catalytic roles for Zap70 in formation of the immunological synapse in CTL. eLife 3:e01310 (2014).
de la Roche, M. et al. Hedgehog signaling controls T-cell killing at the immunological synapse. Science 342, 1247–1250 (2013).