Tauopathies are neurodegenerative diseases which show intracellular accumulation and aggregation of tau protein. In diseases such as Pick's disease, progressive supranuclear palsy, corticobasal degeneration, and argyrophilic grain disease, tau is believed to be the primary disease driver. Additionally, there are several secondary tauopathies, such as Alzheimer’s disease, where the tau accumulation appears to be secondary to a distinct upstream cause, but nevertheless is believed to be an important driver of neurodegeneration.
Due to the high incidence of these diseases, there is a pressing need to develop effective pharmacological therapies.
To accelerate drug discovery, multiple compound libraries which include clinically-approved drugs have been assembled, as such drugs have the potential to be repurposed for novel indications. Because they have been previously well studied in preclinical and human contexts, they can proceed through the drug discovery pipeline more rapidly.
Most large compound screens to identify tauopathy modulators have been performed in cell culture, usually biased towards cancer cell lines. However, such models do not faithfully recapitulate the in vivo environment with its diversity of cell types. In this new paper co-first-authored by Ana Lopez and Farah Siddiqi from the Rubinsztein lab and their zebrafish group led by Angeleen Fleming, a vertebrate model where there was overt toxicity but which was also amenable to moderate-throughput screening was used: a transgenic zebrafish model of tauopathy expressing human wildtype tau in the fish retina. As a consequence of tau-derived toxicity, retinal cells expressing the transgene degenerate in the course of a few days, making it a suitable model to investigate modulators of tau pathology in vivo.
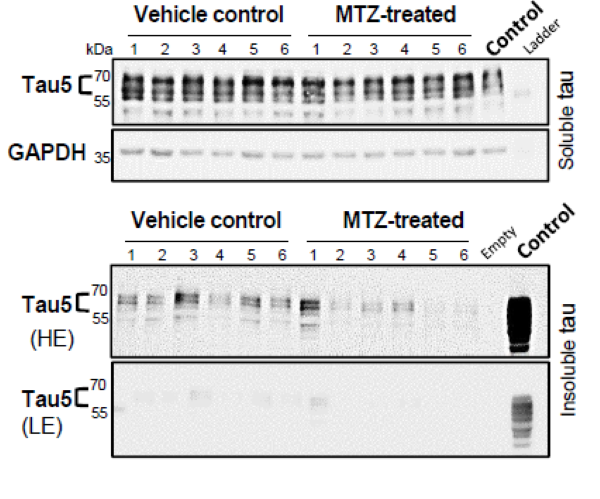
Researchers tested over 1400 clinically-approved compounds using this model. This revealed that carbonic anhydrase (CA) inhibitors protected against tau toxicity. CRISPR experiments confirmed CA depletion mimicked the effects of these drugs. CA inhibition promoted faster clearance of human tau by promoting lysosomal exocytosis. Importantly, methazolamide, a CA inhibitor used in the clinic, also reduced total and phosphorylated tau levels, increased neuronal survival and ameliorated neurodegeneration in mouse tauopathy models at concentrations similar to those seen in people. These data underscore the feasibility of in vivo drug screens using zebrafish models and suggest serious consideration of CA inhibitors for treating tauopathies.

