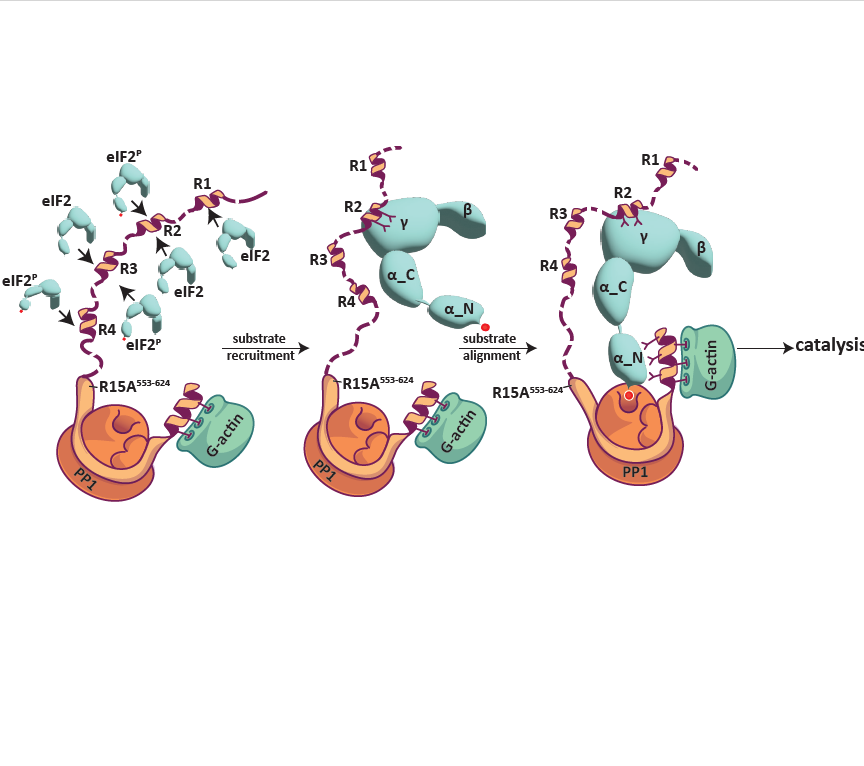
Protein phosphorylation activates important biological processes that are later deactivated by dephosphorylation. Phosphoserine/threonine dephosphorylation is catalyzed by holophosphatases comprising a catalytic subunit, specialized in hydrolytic phosphate removal and regulatory subunit(s) that select phosphoprotein substrates. Dynamics of (de)phosphorylation of phosphoserine 51 on the alpha subunit of eukaryotic translation initiation factors 2 (eIF2) regulates protein synthesis in stressed cells. Previous research has focused on mechanisms operating near the catalytic site of the eIF2-directed holophosphatase. In this paper from the Ron lab, computational, crystallographic, biochemical, and cellular techniques uncover interactions between elements distant from the catalytic site of the eIF2 holophosphatase and its cognate multisubunit eIF2 phosphoprotein substrate. These interactions reveal physiologically important action-at-a-distance that facilitates phosphate removal from eIF2 to efficiently terminate signaling in a mammalian stress response.

