Hereditary spastic paraplegia (HSP) is a group of genetic neurodegenerative conditions characterised by distal axonal degeneration of the corticospinal tract axons. Mutation of the ATL1 gene is one of the most common causes of the disease and encodes the protein Atlastin-1, one of three mammalian atlastins. These homologous dynamin-like GTPases control endoplasmic reticulum (ER) morphology by fusing tubules to form the three-way junctions that characterise ER networks. However, it is not clear whether atlastin-1 is required for correct ER morphology in human neurons and if so what the functional consequences of lack of atlastin-1 are.
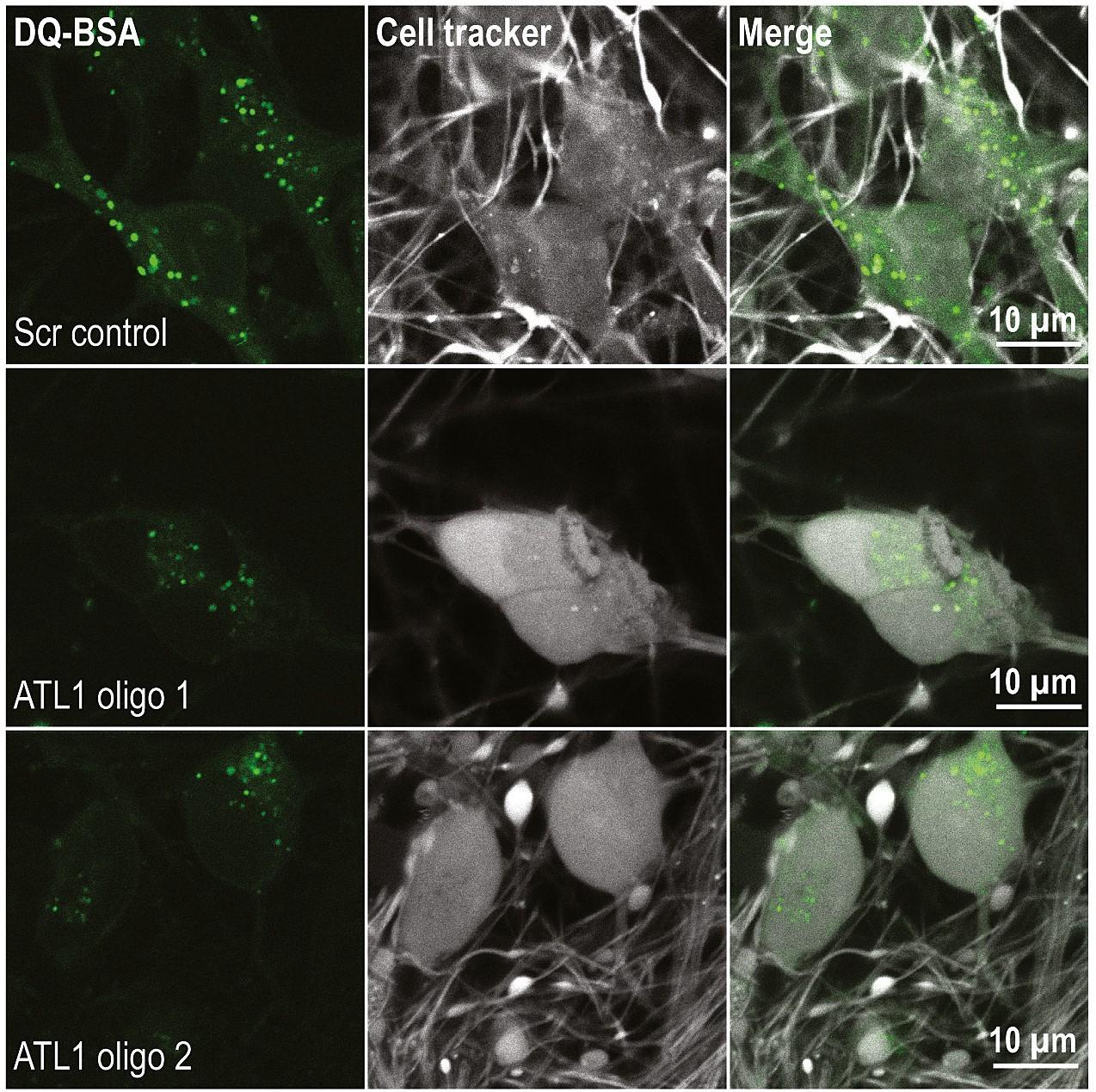
In this study from the Reid lab, CRISPR-inhibition was used to generate human cortical neurons lacking atlastin-1. It was demonstrated that ER morphology was altered in these neurons, with a reduced number of three-way junctions. Neurons lacking atlastin-1 had longer endosomal tubules, suggestive of defective tubule fission. This was accompanied by reduced lysosomal proteolytic capacity.
These results indicate that lack of a classical ER-shaping protein such as atlastin-1 may cause altered endosomal tubulation and lysosomal proteolytic dysfunction. Furthermore, they strengthen the idea that defective lysosome function contributes to the pathogenesis of a broad group of HSPs, including those where the primary localisation of the protein involved is not at the endolysosomal system.

