Chronic liver disease (CLD) leading to cirrhosis accounts for 1 in 25 deaths globally. Significant numbers of CLD cases relate to germline genetic disorders, including haemochromatosis and alpha-1 anti-trypsin (A1AT) deficiency. In A1AT deficiency, inherited mutations of the SERPINA1 gene encode pathogenic forms of the A1AT protein, including the Z-variant which accounts for 95% of sever deficiency. Unlike the wild-type protein, which is secreted into the serum, the Z-variant polymerises in the hepatocyte endoplasmic reticulum (ER), which can lead to ER dysfunction and liver disease. There are currently no treatment options for patients with homozygous germline Z variants that develop liver disease, other than liver transplantation.
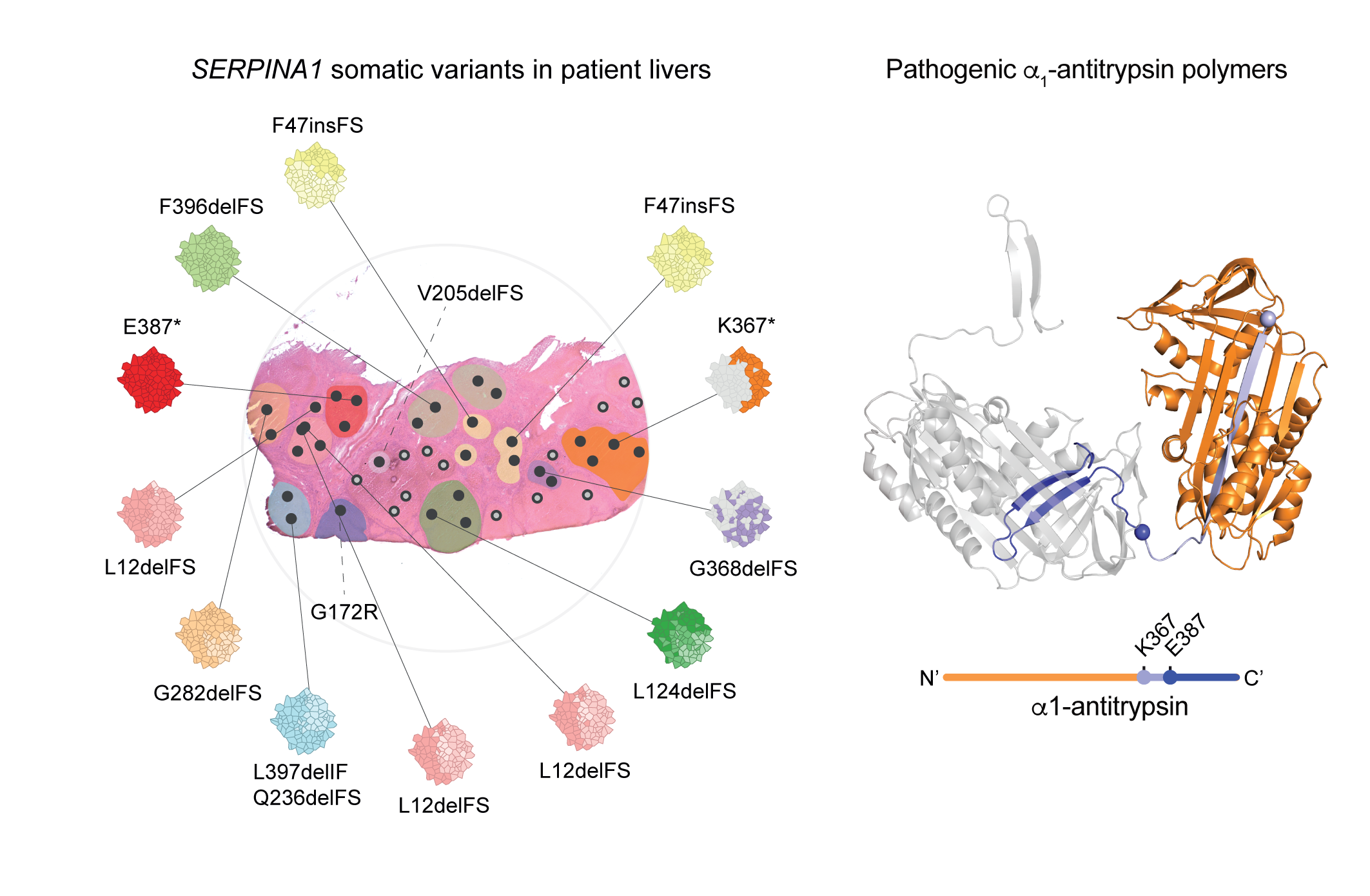
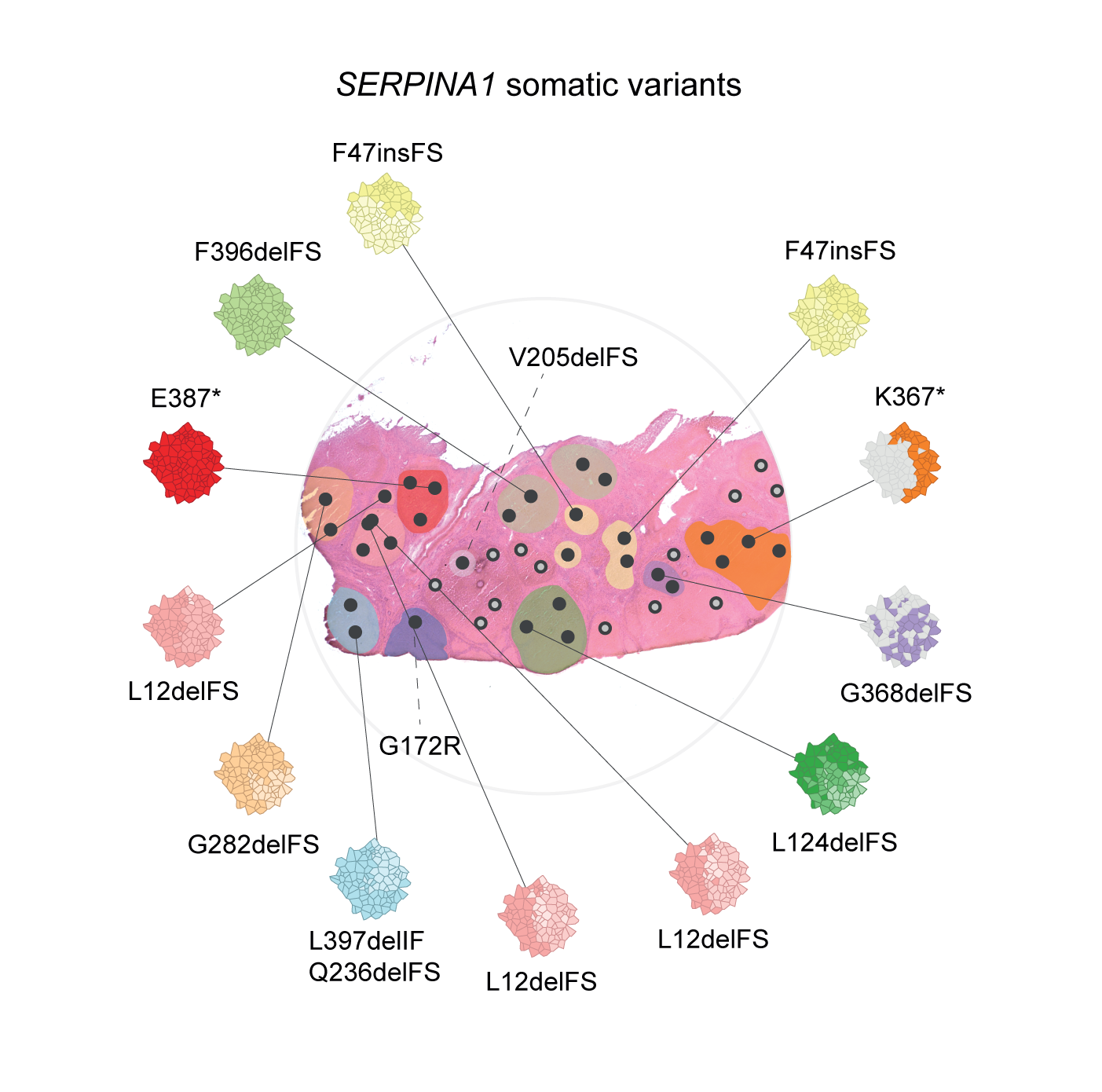
Somatic gene variants have been shown to accumulate in the liver of patients with acquired chronic liver disease, providing clonal advantage to hepatocytes . In this study, we set out to establish whether the accumulation of somatic variants is disease specific. We explored somatic variants and clonal dynamics in the liver of patients with end-stage CLD caused by haemochromatosis and A1AT deficiency. We identified that somatic variants in SERPINA1, are strongly selected for in A1AT deficiency, with evidence of convergent evolution. SERPINA1 somatic variants clustered in the region of the A1AT protein predicted to drive pathogenic polymer formation, mitigating the effects of the germline Z-mutation.
Therefore, somatic escape variants from a pathogenic germline variant are selected for in A1AT deficiency, suggesting adaptive functional somatic variants are disease-specific in CLD and point to disease-associated mechanisms.