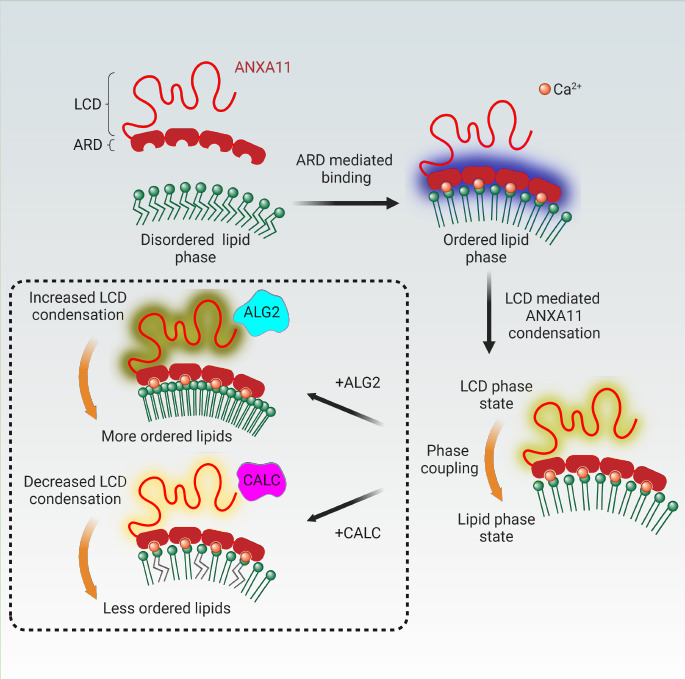
Phase transitions of cellular proteins and lipids play a key role in governing the organisation and coordination of intracellular biology. Recent work has raised the intriguing prospect that phase transitions in proteins and lipids can be co-regulated. In this collaborative paper led by Jonathon Nixon-Abell, we investigated this possibility in the ribonucleoprotein (RNP) granule-ANXA11-lysosome ensemble, where ANXA11 tethers RNP granules to lysosomal membranes to enable their co-trafficking. We show that changes to the protein phase state within this system, driven by the low complexity ANXA11 N-terminus, induces a coupled phase state change in the lipids of the underlying membrane. We identify the ANXA11 interacting proteins ALG2 and CALC as potent regulators of ANXA11-based phase coupling and demonstrate their influence on the nanomechanical properties of the ANXA11-lysosome ensemble and its capacity to engage RNP granules. The phenomenon of protein-lipid phase coupling we observe within this system serves as a potential regulatory mechanism in RNA trafficking and offers an important template to understand other examples across the cell whereby biomolecular condensates closely juxtapose organellar membranes.
In summary, our study provides new insights into a previously unrecognised mechanism for functional crosstalk between biomolecular condensates and their associated organellar membrane partners. It will be of great interest to discover whether other biomolecular condensates alter the lipid organisation and functional properties of the diverse group of membrane-bound organelles across eukaryotic cells.

