Parkinson's disease (PD) is the second most prevalent neurodegenerative disorder, characterised by motor dysfunction, neuronal loss in different regions of the brain, and the accumulation of α-synuclein (α-Syn) aggregates. Currently, there are no effective disease-modifying treatments for PD.
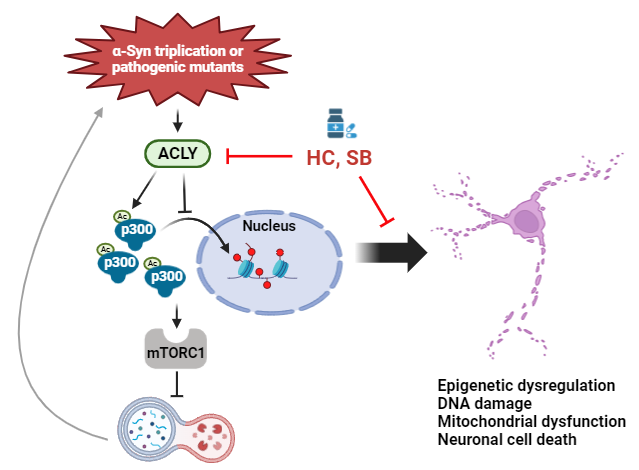
Triplications and certain point mutations in the gene which encodes alpha-synuclein, SNCA, cause PD. In this new paper from the Rubinsztein lab, Sung Min Son and colleagues demonstrate that the PD-causing A53T and triplication α-Syn mutations hyperactivate ATP citrate synthase (ACLY), which impacts two signalling cascades converging to stimulate mTORC1. This impairs autophagy, causing further α-Syn accumulation - Rubinsztein’s lab had shown more than 20 years ago that α-Syn is cleared by autophagy.
Importantly, these studies identify that ACLY may be a suitable therapeutic target, since ACLY inhibitors ameliorated disease signs in iPSC-derived neurons, zebrafish and mouse models of Parkinson's disease caused by the A53T α-Syn mutation.

