Cells are challenged with a variety of stressful situations, one being the accumulation of unfolded proteins, which disrupt normal cellular functions and contribute to diseases like neurodegeneration, diabetes, and cystic fibrosis. To combat this, cells activate the Unfolded Protein Response (UPR), a specialised pathway designed to restore balance by repairing or eliminating these harmful proteins. This process is triggered by specific sensor proteins that act as switches to initiate the stress response.
The study by Neidhardt and colleagues from the Ron lab provides insights into a crucial aspect of cellular stress management: the initial detection of stress from unfolded proteins, the first step in the cascade, by the key sensor protein IRE1. Recognised as a stress sensor since its discovery thirty years ago, the exact way IRE1 identifies stress has been a subject of debate.
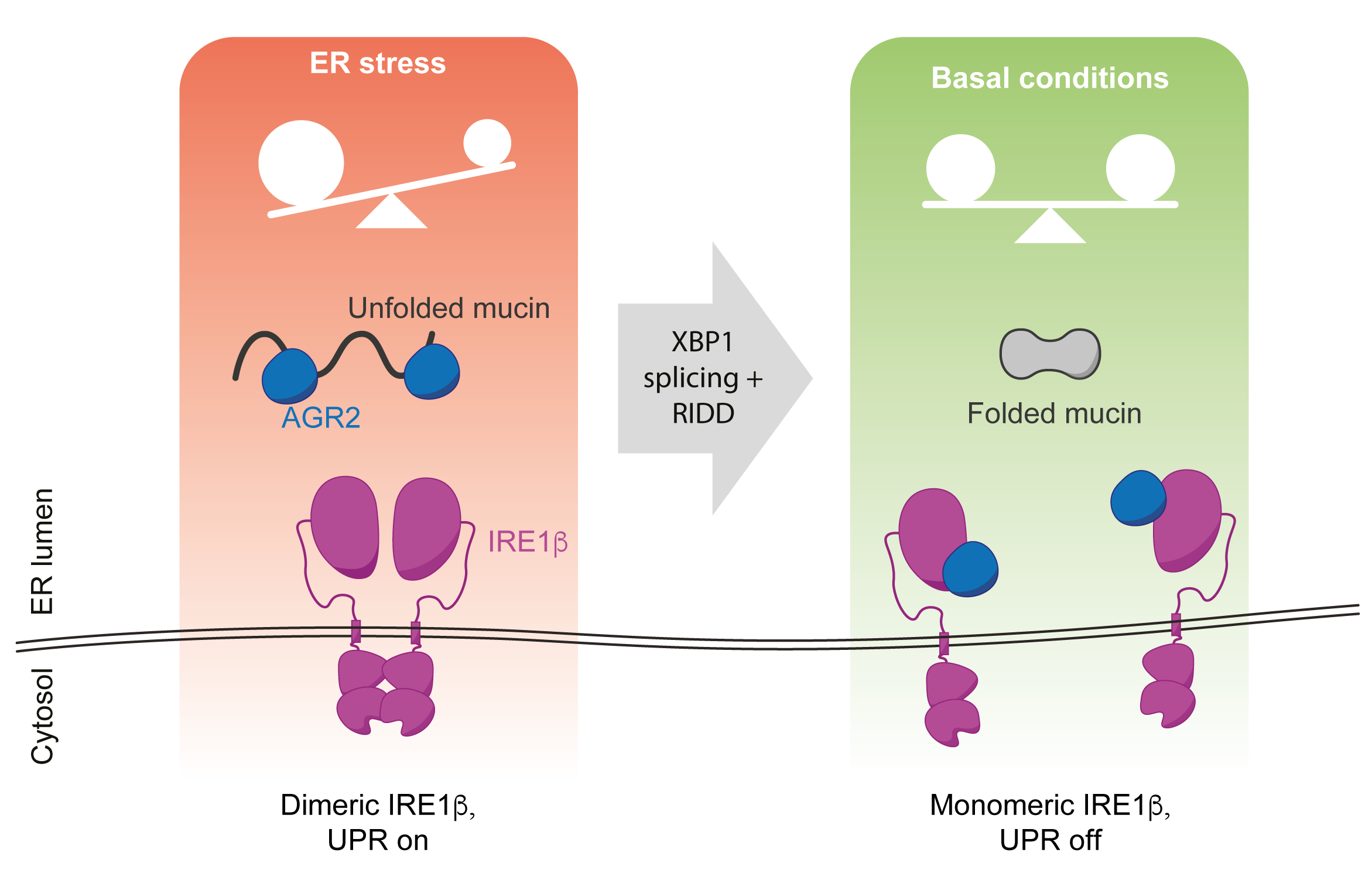
The study focuses on a specific variant of IRE1, known as IRE1b, which is found in mucin-producing cells (the mini-factories that create protective layers in our intestines and airways). It identifies the mucin chaperone AGR2 as a novel inhibitor of IRE1b's stress signalling. This indicates that IRE1b's stress response is tied to mucin levels, making IRE1b a sensor specifically attuned to the demands of mucin production.
Importantly, AGR2 serves a dual role: it both manages the stress response and oversees mucin production, establishing a critical link between these two cellular processes. This discovery not only illuminates IRE1b's unique function in mucin-producing cells but also supports the broader idea that cellular stress sensing is intertwined with protein folding, mediated by a chaperone that manages both stress response and protein folding.

