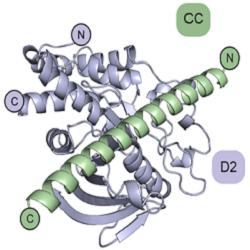
The labs of Dr Janet Deane at CIMR and Dr Hayley Sharpe at the Babraham Institute work collaboratively to understand the structures and substrate specificities of certain receptor protein tyrosine phosphatases (PTPRs). They had previously reported a series of substrates for one such enzyme, PTPRK, which supports cell adhesion and has been implicated as a tumour suppressor. In a follow-up publication in eLife, PhD student Iain Hay and colleagues identified a docking site in the catalytically inactive D2 pseudophosphatase domain that promotes substrate dephosphorylation by the D1 phosphatase domain. This is the first example of what seems likely to be a general principle for phosphotyrosine substrate recruitment through docking sites on catalytically inactive D2 domains. We know very little about how PTPs select their substrates and this discovery of a substrate docking site on the PTPRK D2 domain is a significant advance in the receptor tyrosine phosphatase field. Importantly, this suggests that the D2 pseudophosphatase domains of other RPTPs may similarly function in substrate recruitment and selectivity.

