Deregulated mTOR signaling is implicated in many diseases, including neurodegeneration, as well as aging. Hutchinson-Gilford progeria syndrome (HGPS) is a rare sporadic autosomal dominant accelerated aging disorder. At 9-24 months, affected individuals show striking growth delay and develop a characteristic appearance. The condition manifests with numerous ageing features, including decreased skin elasticity, hair loss and coronary artery disease which typically causes death in the early teens.
The mechanistic target of rapamycin complex 1 (mTORC1) is a master regulator of cell growth, metabolism and autophagy. This paper from Sung Min Son in David Rubinsztein’s lab, in collaboration with Delphine Larrieu’s group, describes that depletion of various amino acids (like leucine) or glucose causes cytoplasm-to-nuclear shuttling of p300, which reduces mTORC1 activity and activates autophagy. p300 cytoplasm-nucleus shuttling is compromised in cells from patients with HGPS, causing mTORC1 hyperactivation and defective autophagy. These phenotypes and a number of other defects seen in HGPS cells were normalised by modulating p300 nucleocytoplasmic shuttling.
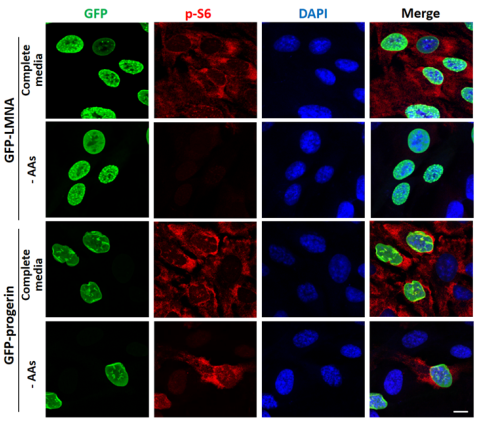
Image: mTORC1 activity (assessed by p-S6 staining) is dramatically reduced by amino acid-depletion in wild-type cells (GFP-LMNA) but not in cells expressing the HGPS mutation (GFP-progerin).

