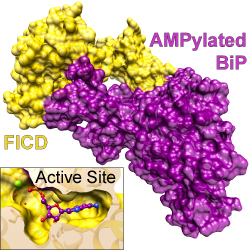
BiP is a molecular chaperone of the endoplasmic reticulum (ER), playing a tightly-regulated role in protein folding and the Unfolded Protein Response. Under normal conditions, a substantial fraction of BiP exists in an inactive, covalently AMPylated form. Upon the occurrence of ER stress, this pool of AMPylated BiP is converted back into its active state through de-AMPylation. Unusually, a single enzyme, FICD, is responsible for both of these antagonistic processes: monomeric FICD preferentially catalyses BiP AMPylation while dimeric FICD preferentially acts as the de-AMPylase. Luke Perera and colleagues from David Ron’s lab, together with Nathan Zaccai and the neutron scattering facility at Institut Laue-Langevin (Grenoble) have conducted a detailed structural analysis of a deAMPylation complex of FICD and AMPylated BiP. Published in Nature Communications, their paper provides new insight into the mechanism of FICD-mediated regulation of BiP.

