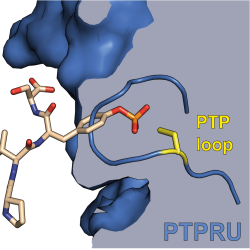
Reversible protein tyrosine phosphorylation by kinases and phosphatases is a key component of cellular signalling. CIMR PhD student Iain Hay and colleagues from the labs of co-supervisors Janet Deane (CIMR) and Hayley Sharpe (Babraham), together with Maja Koehn (Freiburg) have published in Nature Communications on the structure and function of the receptor protein tyrosine phosphatase PTPRU. X-ray crystallography and biochemical analysis reveal a novel mechanism by which PTPRU regulates tyrosine phosphorylation indirectly through its two catalytically inactive phosphatase domains. By competing with related, active receptor tyrosine phosphatases, PTPRU acts to ‘protect’ the phosphotyrosines of shared substrates. This process is likely to be important for fine-tuning the formation and stability of cell-cell junctions.

