Soday L, Ravenhil BJ, Houghton J, Williamson JC, Antrobus R, Matheson NJ, Weekes MP. Comparative cell surface proteomic analysis of the primary human T cell and monocyte responses to type I interferon. 2021. Frontiers in Immunology. 12:600056.
Lin K, Nightingale K, Soday L, Antrobus R, Weekes MP. Rapid degradation pathways of host proteins during HCMV infection revealed by quantitative proteomics. 2021. Frontiers in Cellular and Infection Microbiology. 10:578259.
Rivett L, Sridhar S, Sparkes D, Routledge M, Jones N, …., Matheson NJ, Wright G, Goodfellow I, Baker S, Weekes MP. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. 2020. eLife. 9:e58728.
Fletcher-Etherington A, Nobre L, Nightingale K, Antrobus R, Nichols J, Davison AJ, Stanton RJ, Weekes MP. Human cytomegalovirus protein pUL36: a dual cell death pathway inhibitor. 2020. PNAS. 117:18771-18779.
Jones NK, Rivett L, Sparkes D, Forrest S, Sridhar S, Young J, …, Wright G, Matheson NJ, Baker S, Weekes MP. Effective control of SARS-CoV-2 transmission between healthcare workers during a period of diminished community prevalence of COVID-19. 2020. eLife.
Soh TK, Davies CTR, Muenzner J, Connor V, Smith C, Bouton CR, Emmott E, Graham SC*, Weekes MP*, Crump CM*. Herpes simplex virus-1 pUL56 degrades GOPC to alter the plasma membrane proteome. 2020. Cell Reports. 33:108235.
Ravenhill BJ, Soday L, Houghton J, Antrobus R, Weekes MP. Comprehensive cell surface proteomics defines markers of classical, intermediate and non-classical monocytes. 2020. Scientific Reports. 10 (1): 4560.
Nobre L, Nightingale K, Ravenhill B, Antrobus R, Soday L, Nichols J, Wang ECY, Davison AJ, Wilkinson GWG, Stanton RJ, Huttlin EL, Weekes MP. Human cytomegalovirus interactome analysis identifies degradation hubs, domain associations and viral protein functions. eLife. 2019.
Soday L, Lu Y, Albarnaz JD, Antrobus R, Smith GL*, Weekes MP*. Quantitative temporal proteomic analysis of vaccinia virus infection reveals regulation of histone deacetylases by an interferon antagonist. 2019. Cell Reports. 7:1920-1933. *Joint last authorship.
Ravenhill BJ, Kanjee U, Ahouidi A, Nobre L, Williamson J, Goldberg JM, Antrobus R, Dieye T, Duraisingh MT, Weekes MP. Quantitative comparative analysis of human erythrocyte surface proteins between individuals from two genetically distinct populations. 2019. Communications Biology. 2(350):1-9.
Wang LW, Shen h, Nobre L, Ersing I, Paulo JA, Trudeau S, Sommermann T, Ma Y, Reinstadler B, Nomburg J, Cahir-McFarland E, Gygi SP, Mootha VK, Weekes MP*, Gewurz BE*. Epstein-Barr Virus Induced One-Carbon Metabolism Drives B-Cell Transformation. 2019. Cell Metabolism. 30:539-555. *Joint last authorship.
Caller LG, Davies CTR, Antrobus R, Lehner PJ, Weekes MP*, Crump CM*. Temporal proteomic analysis of BK polyomavirus infection reveals virus-induced G2 arrest and highly effective evasion of innate immune sensing. 2019. J. Virol. 93(e00595-19):1-21.
Wei Wang L, Wang Z, Ersing I, Nobre L, Trudeau S, Zhao B, Weekes MP, Gewurz BE. Epstein-Barr virus subverts mevalonate and fatty acid pathways to promote infected B-cell proliferation and survival. 2019. PLOS Pathogens. 15(e1008030):1-35.
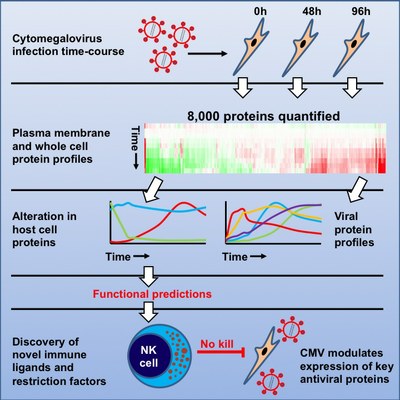
Nightingale K, Lin KM, Ravenhill B, Ruckova E, Davies C, Nobre L, Fielding CA, Fletcher-Etherington A, Soday L, Nichols H, Sugrue D, Wang ECY, Moreno P, Umrania Y, Antrobus R, Davison AJ, Wilkinson GWG, Stanton RJ , Tomasec P, Weekes MP. High definition analysis of host protein stability during human cytomegalovirus infection reveals antiviral factors and viral evasion mechanisms. 2018. Cell, Host & Microbe. 24:447-460.